Which Of The Following Signs On Q And W Represent A System
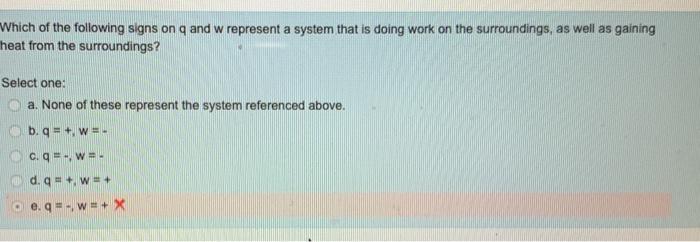
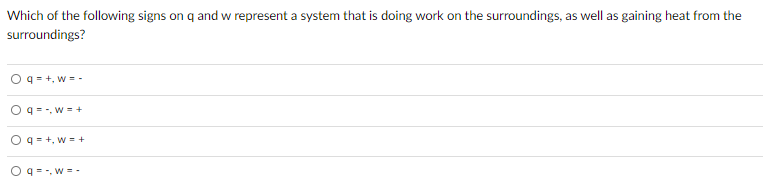
Which of the following signs on q and w represent a system. Work w is categorized by net in as positive work done on the systemhence Work done by the system is negative. Get the detailed answer. 23Which of the following signs on q and w represent a system that is doing work on the surroundings as well as gaining heat from the surroundings.
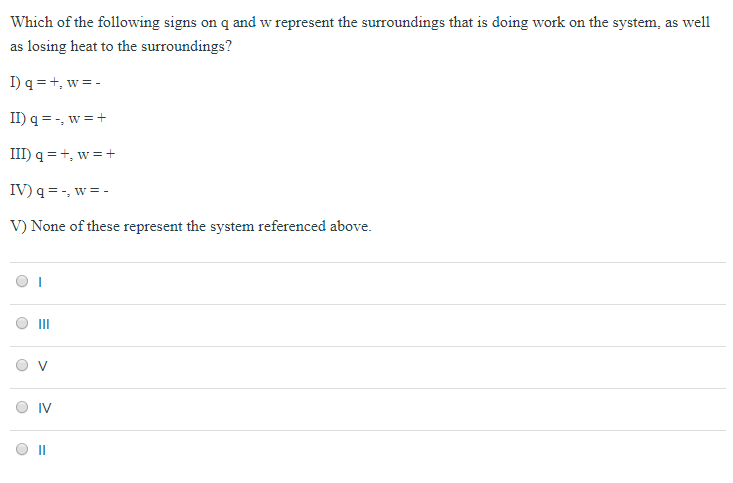
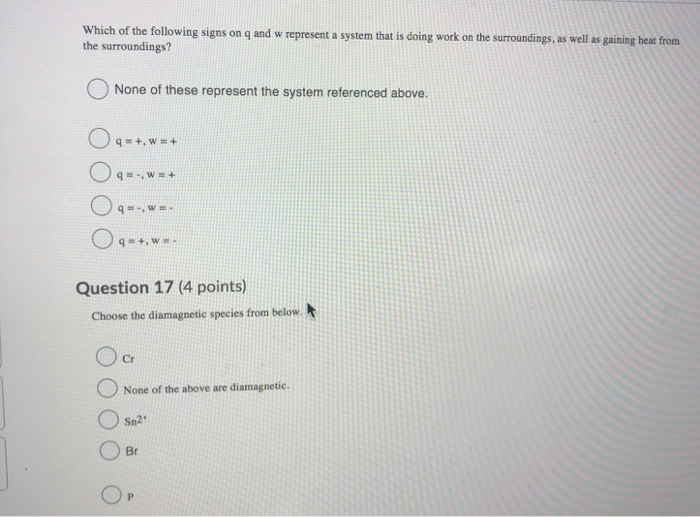
Which of the following signs on q and w represent a system that is doing work on the surroundings as well as gaining heat from th. 2 on a question Which of the following signs on q and w represent a system that is doing work on the surroundings as well as gaining heat from the surroundings. 3 question Which of the following signs on q and w represent a system that is doing work on the surroundings as well as losing heat to the surroundings.
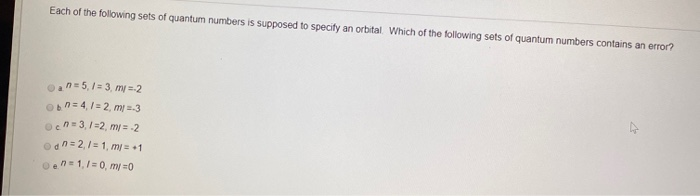
How many orbitals are contained in the third principal level n 3 of a given atom. Q - w -. Which of the following signs on q and w represent a system that is doing work on the surroundings as well as losing heat to the surroundings.
Aq w b q - w c q w dq - w e None of these represent the system referenced above. A q w b q w c q w d q w e None of these represent the system described above. 3 5 9 18.
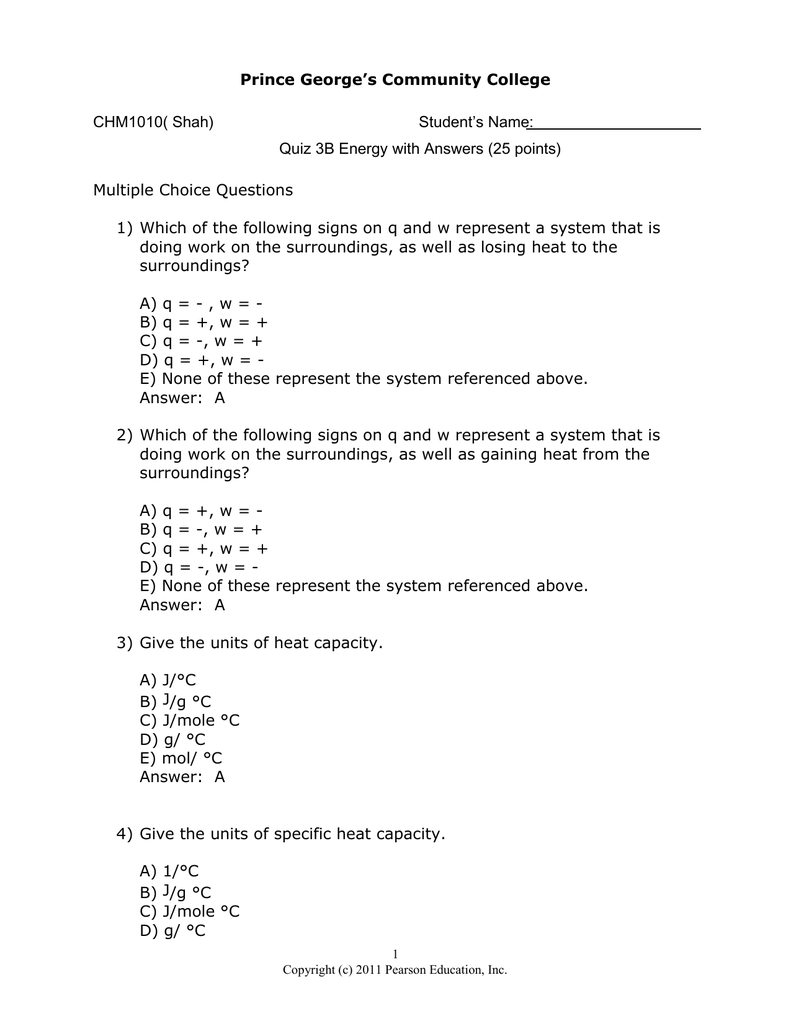
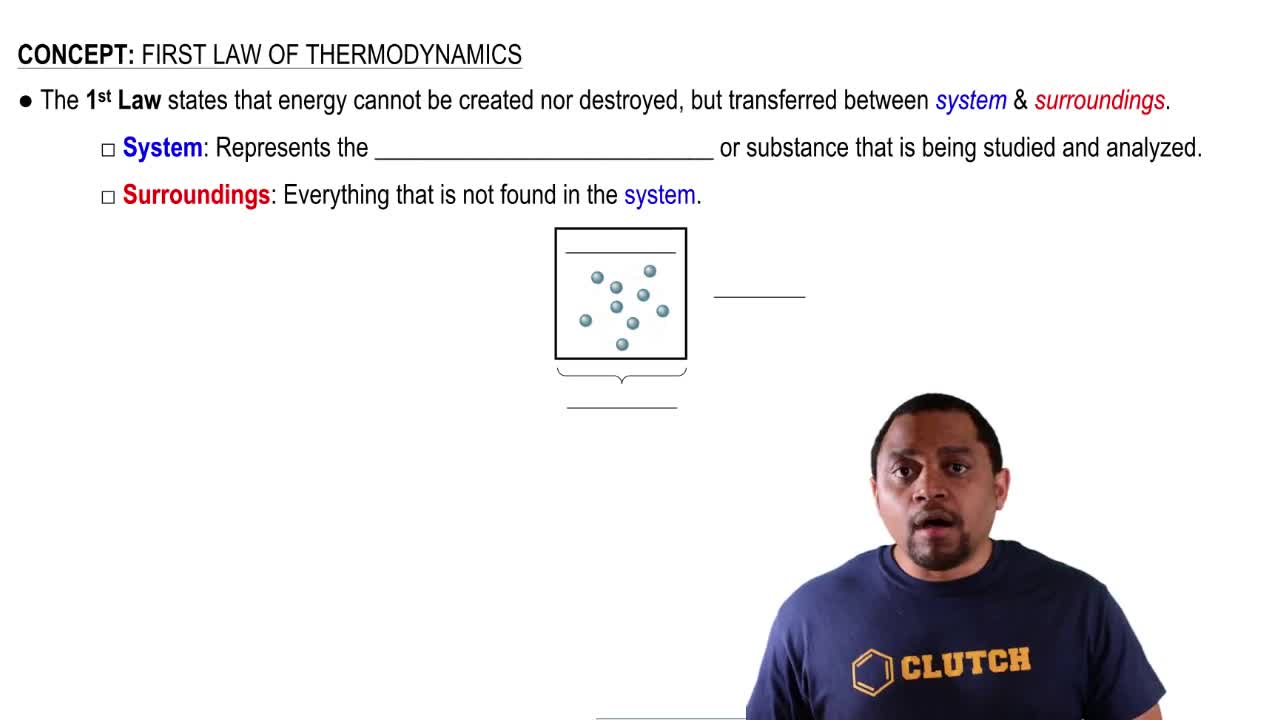
Which of the following signs on q and w represent a system that is doing work on the surroundings as well as losing heat to the surroundings. Heat transfer q is categorized by net in as positive Heat transfer in to the systemhence Heat loss from the system is negative. Enthalpy of Hydration Quiz Instructions Which of the following signs on q and w represent a system that is doing work on the surroundings as well as losing heat to the surroundings.
Which of the following signs on q and w represent a system that is doing work on the surroundings as well as losing heat to the surroundings. A q - w - B q w C q - w D q w - E None of these represent the system referenced above. Q - w - Which of the following signs on q and w represent a system that is doing work on the surroundings as well as gaining heat from the surroundings.
Asked Jul 27 2019 in Chemistry by csims4551 A. Which of the following signs on q and w represent a system that is doing work on the surroundings as well as losing heat to the surroundings.
A q - w - b q w c q - w d q w - e none of these represent the system referenced above.
3 5 9 18. Aq w b q - w c q w dq - w e None of these represent the system referenced above. Heat transfer q is categorized by net in as positive Heat transfer in to the systemhence Heat loss from the system is negative. How many orbitals are contained in the third principal level n 3 of a given atom. Which of the following signs on q and w represent a system that is doing work on the surroundings as well as gaining heat from th. Enthalpy of Hydration Quiz Instructions Which of the following signs on q and w represent a system that is doing work on the surroundings as well as losing heat to the surroundings. 3 question Which of the following signs on q and w represent a system that is doing work on the surroundings as well as losing heat to the surroundings. Group of answer choices q - w - q w q - w q w - Question 2 If the system loses heat the surroundings _____ heat. A q - w - B q w C q - w D q w - E None of these represent the system referenced above.
Which set of signs for q and w represent a system that is doing work on the surroundings and losing heat to the surroundingsa -q -wb q wc -q wd q -we None of these represent the system referenced above. Which of the following signs on q and w represent a system that is doing work on the surroundings as well as losing heat to the surroundings. Which of the following signs on q and w represent a system that is doing work on the surroundings as well as gaining heat from the surroundings. Aq w b q - w c q w dq - w e None of these represent the system referenced above. Correct answer - Which of the following signs on q and w represent a system that is doing work on the surroundings as well as losing heat to the surrou. How many orbitals are contained in the third principal level n 3 of a given atom. Q - w q w q w - q - w - None of these represent the system referenced above.





































Post a Comment for "Which Of The Following Signs On Q And W Represent A System"